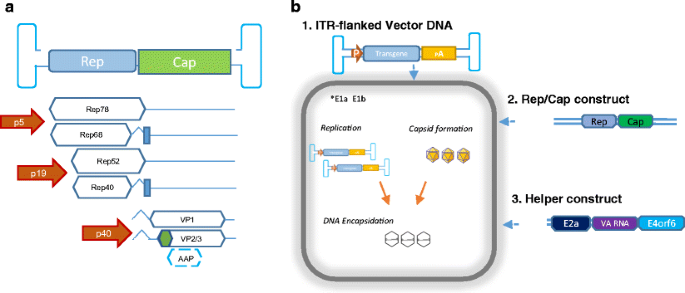
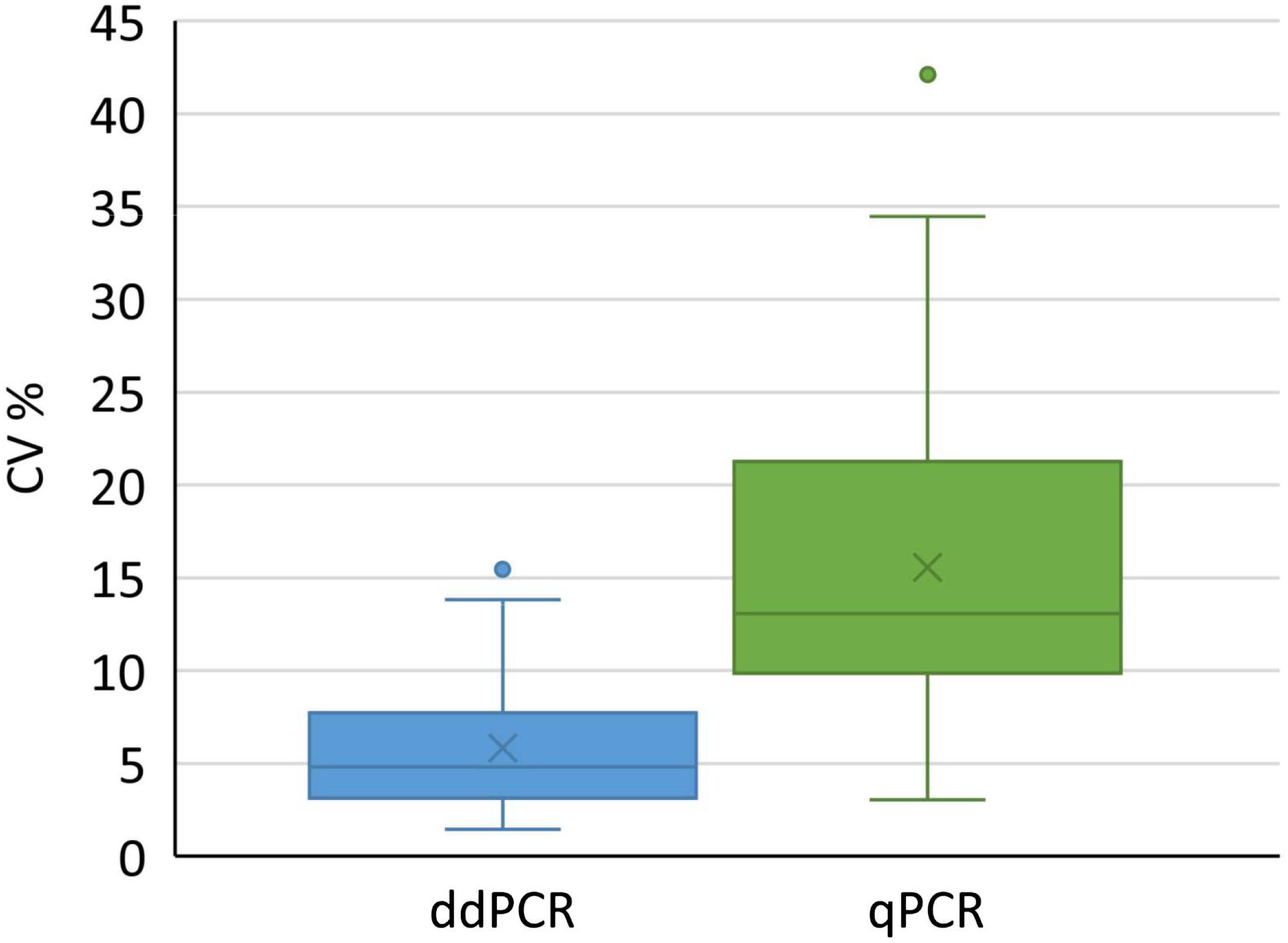
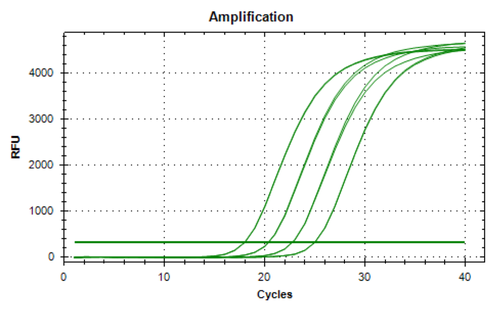
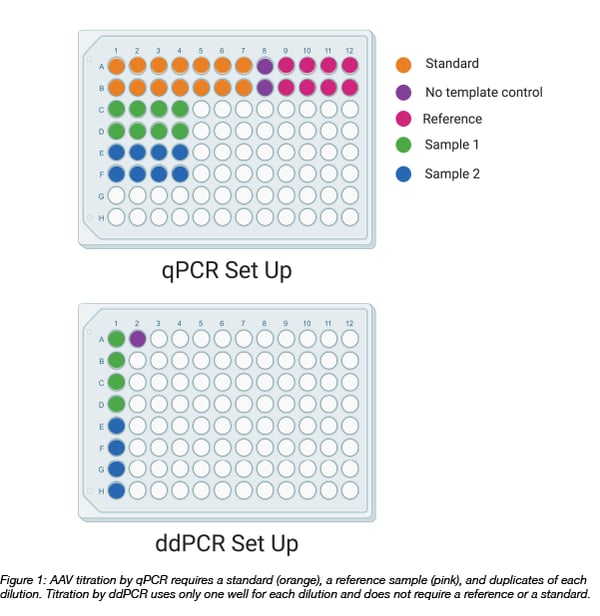
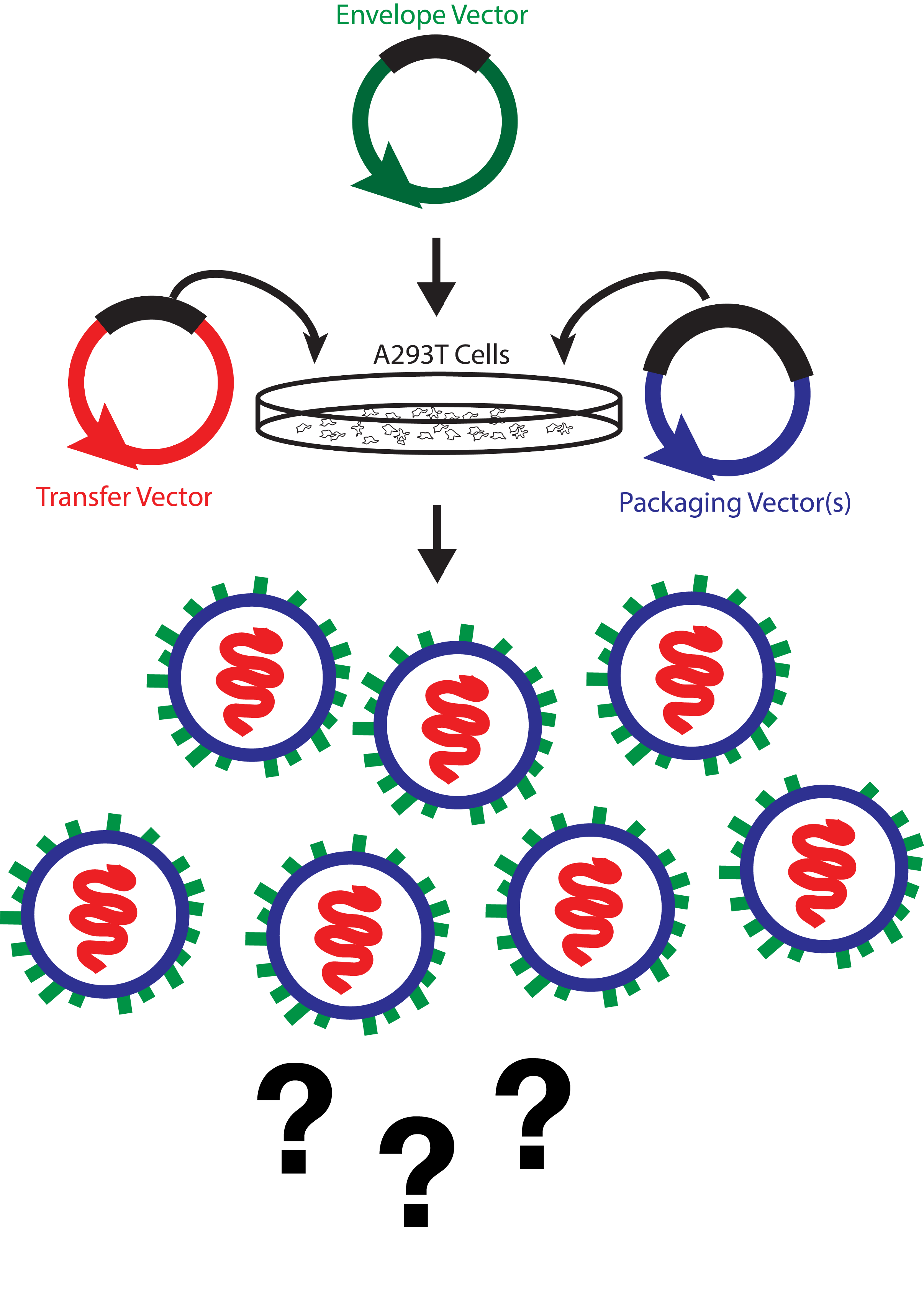
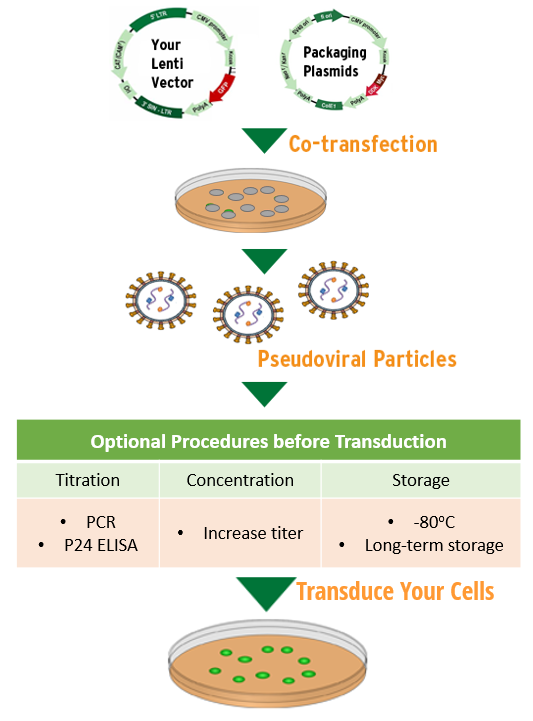
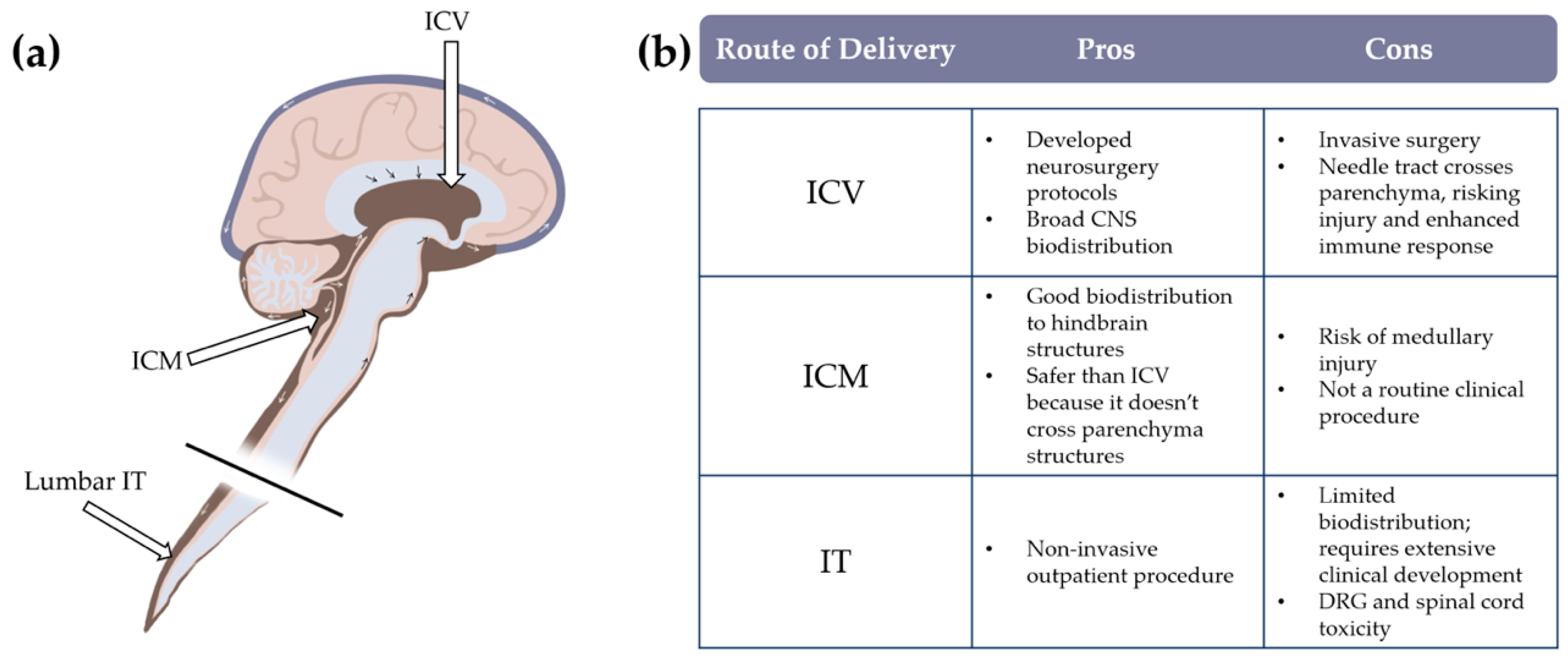
Vector Genome Titer
Aav particles are very stable.

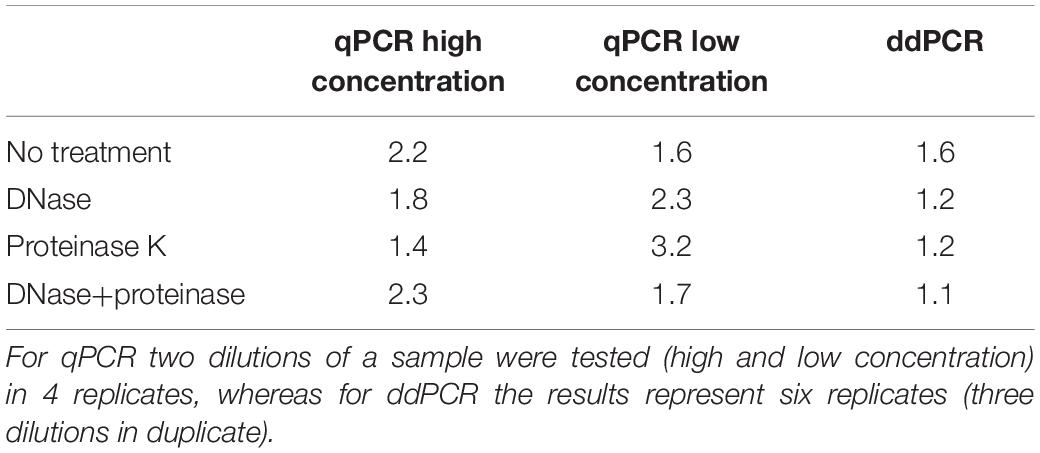
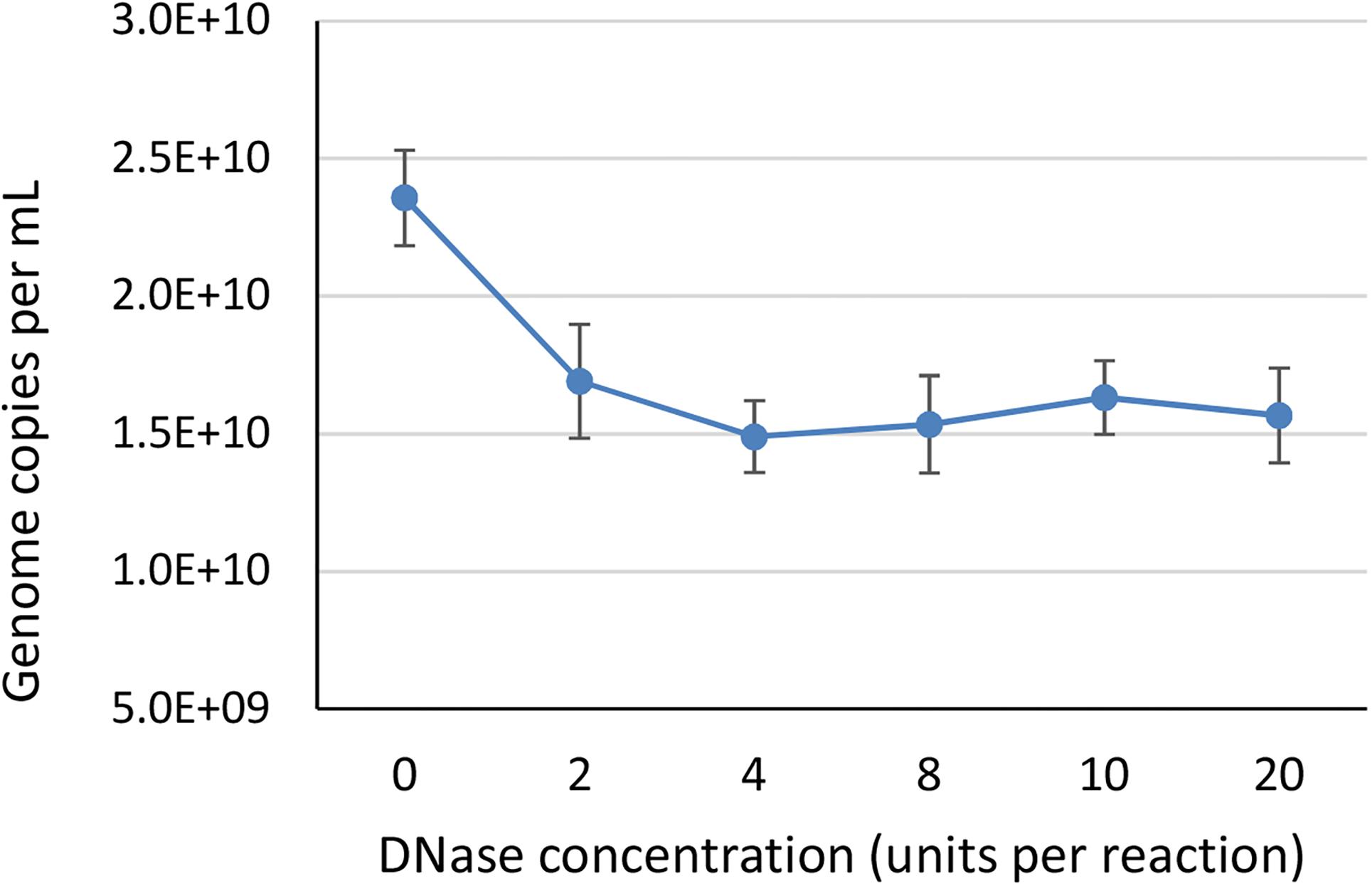
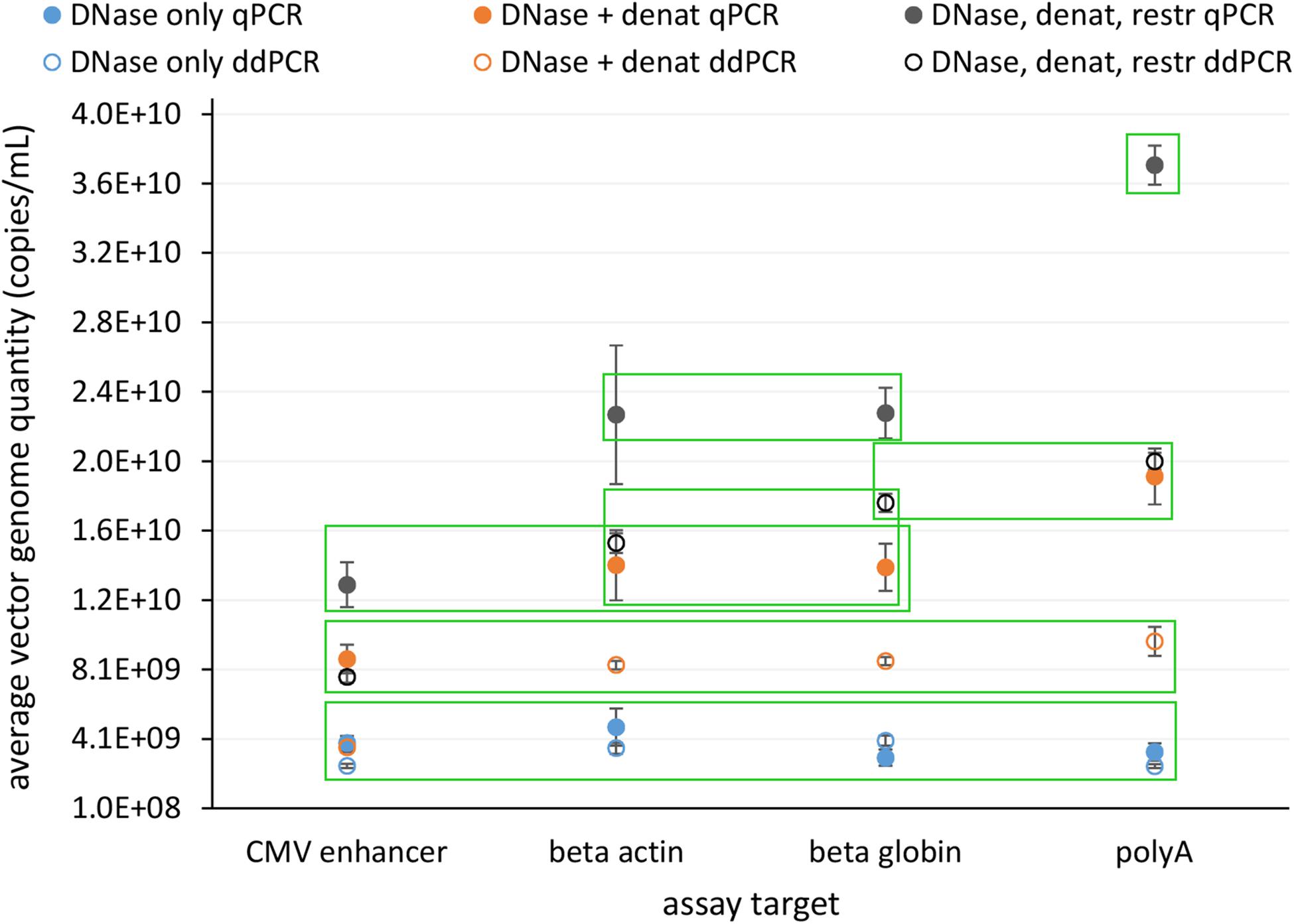
Vector genome titer. Aav titers are given as a physical titer in viral genomes per ml vgml determined by direct qpcr of purified vector particles. For vectors with wild type aav8 capsid elisa total particles and qpcr full recombinant genome containing particles titers were basically the same indicating that they essentially contained particles with encapsidated vg similar to the raav8rsm20in contrast the aav8dvp1 gfp vector stock contained 3 fold more total particles than vg containing particles ie two thirds of the particles with no empty or illegitimate non vector dna encapsidated. Other than a theoretical maximum value physical titers dont necessarily give any indication of the infectious titer. The lowest vector genome titer was determined for samples treated only with dnase.
This same group also included dnase and denaturation treatment with ddpcr cmv assay. For vectors with wild type aav8 capsid elisa total particles and qpcr full recombinant genome containing particles titers were basically the same indi cating that they essentially contained particles with encapsidated vg similar to the raav8rsm20in contrast the aav8dvp1 gfp vector stock contained 3 fold more total particles than vg contain ingparticlesietwo thirdsoftheparticleswithnoemptyorillegit imate non vector dna encapsidated. Physical titers are measured by quantifying the concentration of viral genomes by qpcr or other dna quantification methods see below since each viral particle typically contains one viral genome. This was also the only treatment with no significant differences in the determined vector genome titer over all assays and both pcr techniques figure 4.
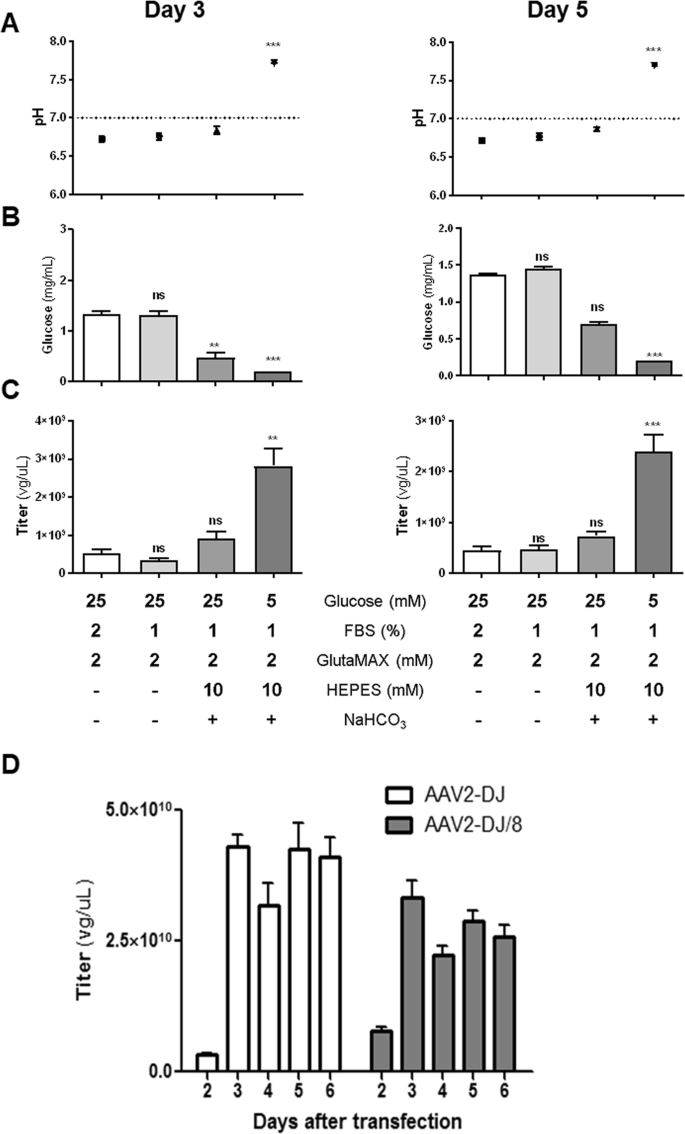
We measure the physical titer of aav by directly extracting viral genome from lysed viral particles and then using qpcr to accurately quantify the copy number of viral genome using the copy number of itr region as a proxy in the stock. Error bars indicate the standard deviations between replicate assays. Large scale manufacture of aav vector for clinical trials requires a high degree of sophistication in both process design and lot release assays. The concentration of viral particles containing viral genomes.
The optical density a 260280 assay measures the concentration of viral dna and protein. Eight lots of aav hfix16 with capsid to vector genome ratios ranging from 1 to 40 and titers ranging from 46 1011 to 1 1013 vgml were analyzed by spectrophotometry. It is a physical assay measuring the concentration of viral particles vp. The calculated vgml based on the absorbance is plotted against the vgml obtained by q pcr.
Further improvement in vector titer and purity will expedite this in vivo exploration and provide preclinical information required for use in human gene therapy. The vector dna signal is compared to the signal from the plasmid dna standard curve and extrapolated to determine a vector genome titer.